Introduction:
Waldenström macroglobulinemia (WM) is a B-cell lymphoproliferative disorder characterized by lymphoplasmacytic bone marrow infiltration and immunoglobulin M (IgM) monoclonal gammopathy. Various studies have shown that the proteasome inhibitor bortezomib (V) targets signaling pathways of relevance in WM. We reported published data on the efficacy profile of (V) for the patients of WM.
Methods:
We performed a comprehensive literature search on articles following PRISMA guidelines. Beginning with articles published after 2004, we used databases like PubMed, Embase, Clinicaltrials.gov, Cochrane Library and Web of Science. Total 580 articles were identified initially and after detailed screening, we finalized 9 studies involving 375 patients with Waldenstrom Macroglobulinemia (WM) treated by combination of Bortezomib.
Results:
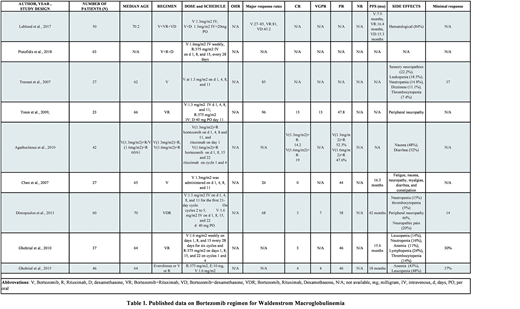
The total number of patients in the studies involving Bortezomib were 375 and dose of the drug used ranged between 1.3 mg/m2 to 1.6mg/m2. The Complete response (CR) ranged from 0-19% and the partial response (PR) ranged from 44-58%.
Bortezomib(V):
In the study Treonet et al., N=27, the highest major respose rate was 85% and minimal response rate was 37%. In the study Chen et al., N=27, the major response rate was 26% and the PR was 44%. Progression free survival (PFS) was 16.3 months for Chen et al.
Bortezomib(V), Rituximab(R), and Dexamethasone(D):
In the study Treon et al., N=23, the highest major response rate was 96%, CR was 13%, Very good partial response (VGPR) was 13%, and PR was 46%. Highest PR and was observed in Dimopolous et al., which was 58% and 42 months respectively.
Bortezomib(V), and Rituximab(R):
In the study Agathocleus et al., N=42, the highest CR was 14.2-19%. The highest PR was between 47.6-52.3%. In the study Ghobrial et al., N=37, the CR was 3% and PR was 46%. PFS was 15.6 months.
Bortezomib(V), Rituximab(R) and Everolimus(E):
In the study Ghobrial et al., N=46, the CR was 4%, PR was 46% and PFS was 18 months.
Conclusion:
Despite heterogenicity in WM patients and various regimens used in literature, Bortezomib containing regimens are a remarkably effective treatment. Combination regimens showed an overall response rate of 96%. The main side effects being anemia, leukopenia, thrombocytopenia, nausea and sensory neuropathies. Although we recommend future randomized prospective trials to better understand the efficacy and safety profile of Bortezomib for WM treatment.
Anwer:Incyte, Seattle Genetics, Acetylon Pharmaceuticals, AbbVie Pharma, Astellas Pharma, Celegene, Millennium Pharmaceuticals.:Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal